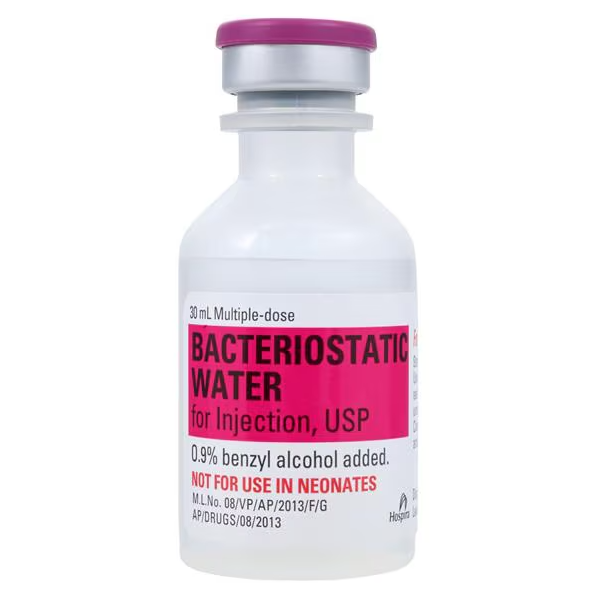
Product Specifications
| Specification | Details |
|---|---|
| Manufacturer | Pfizer Inc. |
| Manufacturer # / NDC | 00409-3977-03 |
| Product Type | Bacteriostatic Water for Injection, USP |
| Strength | 0.9% Benzyl Alcohol added as bacteriostatic preservative |
| Volume | 30 mL Multi-Dose Vial |
| Application | Diluent / solvent for reconstituting or diluting injectable medications |
| Sterility | Sterile |
| Container Type | Glass vial with flip-off cap |
| Route | Intramuscular, intravenous, or subcutaneous (as directed by prescribing info) |
| Shelf Life | 2 years (unopened); use within 28 days once punctured |
| Recommended Storage | Store at 20° to 25°C (68° to 77°F); avoid freezing |
| UNSPSC Code | 51191605 |
Key Features
-
Sterile bacteriostatic water formulated with benzyl alcohol preservative.
-
Intended as a diluent for reconstituting compatible drugs prior to injection.
-
Multi-dose vial allows repeated withdrawals for up to 28 days after opening.
-
USP standard quality manufactured by Pfizer.
-
Essential supply for hospitals, pharmacies, compounding, and clinical use.




Reviews
There are no reviews yet.