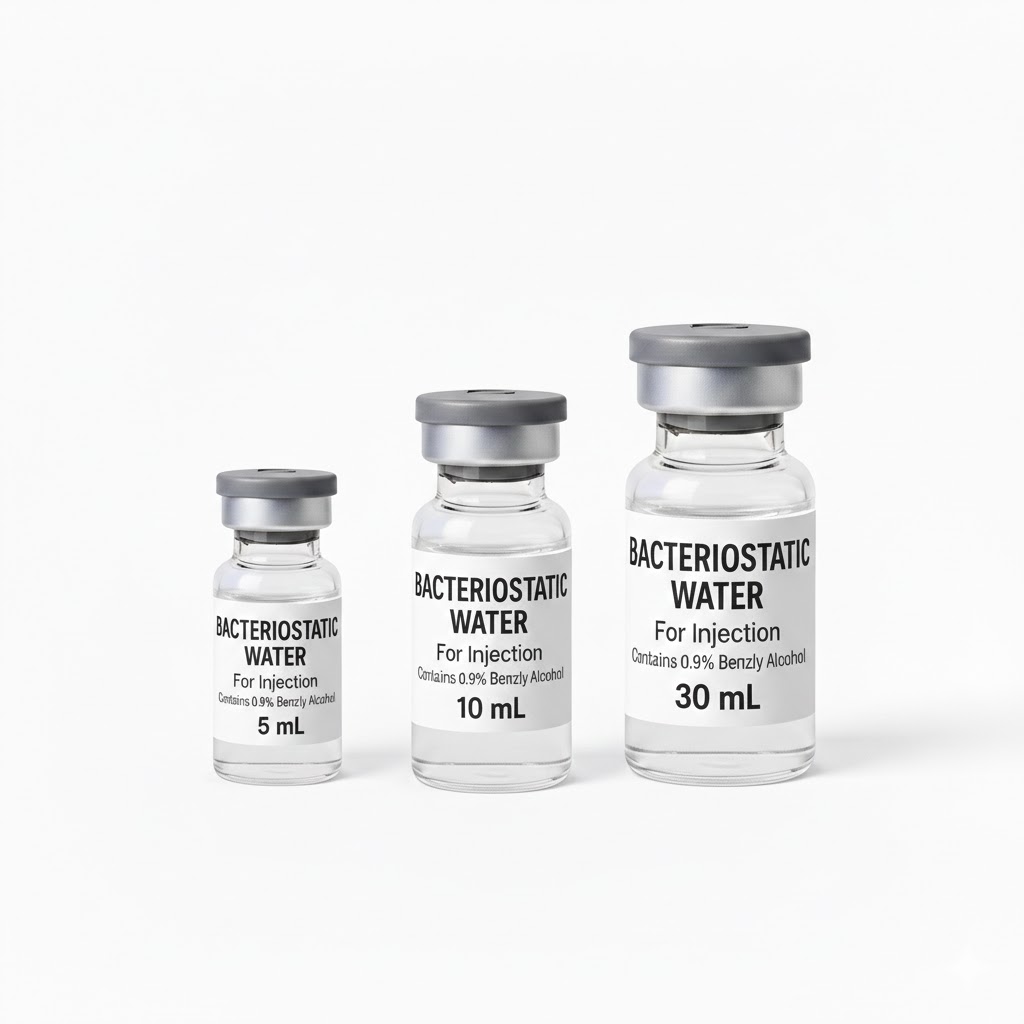
General Product Specifications
| Specification | Details |
| Generic Name | Bacteriostatic Water for Injection, USP |
| Manufacturer | Generic (Hospira/Pfizer or equivalent) |
| Active Ingredient | Sterile Water for Injection |
| Preservative | Benzyl Alcohol 0.9% (9 mg/mL) |
| Sterility | Sterile, Nonpyrogenic |
| Traceability | Lot Number included on every vial |
| Expiration / BUD | 3 Months |
| Storage | 20°C to 25°C (68°F to 77°F) |
Key Features
-
Preservative-Enhanced: Contains 0.9% benzyl alcohol to inhibit bacterial growth, supporting multiple withdrawals for medication reconstitution.
-
Controlled Shelf Life: Adheres to a strict 3-month expiration/BUD protocol to guarantee product stability and preservative strength.
-
Clinical Versatility: Ideal for dissolving or diluting compatible vaccines, peptides, and other injectable medications.
-
Convenient Access: Available in three sizes (5 mL, 10 mL, and 30 mL) to accommodate various clinical volume requirements.
⚠️ Warning: NOT FOR USE IN NEONATES. Benzyl alcohol is associated with “Gasping Syndrome” in newborns. Do not use for fluid replacement or in epidural/spinal procedures.
🌡️ Storage: Store at controlled room temperature of 20°C to 25°C (68°F to 77°F). Discard any vial that is past the 3-month BUD or shows signs of contamination.




Reviews
There are no reviews yet.