Product Specifications
| Specification | Details |
|---|---|
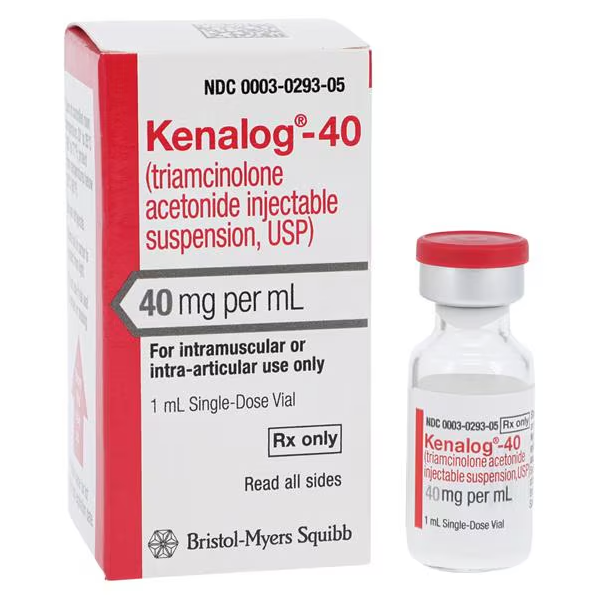
| Manufacturer / Brand | Bristol-Myers Squibb |
| Manufacturer # / SKU | 029305 (NDC 00003-0293-05) |
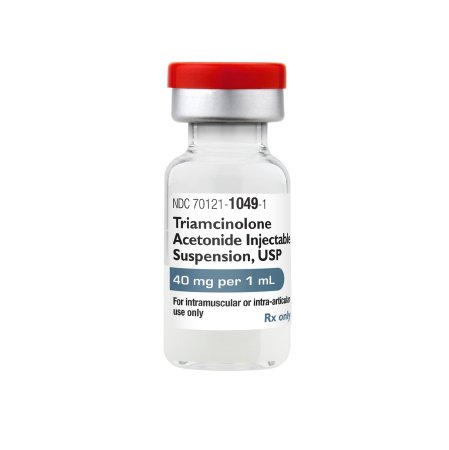
| Product Type | Triamcinolone Acetonide Injectable Suspension |
| Strength / Concentration | 40 mg/mL |
| Volume | 1 mL Single-Dose Vial |
| Dosage Form | Sterile aqueous suspension |
| Route of Administration | Intramuscular or Intra-Articular only |
| Sterility | Sterile |
| Preservative | Contains benzyl alcohol (0.99% w/v) |
| Packaging / Sold As | Each vial (1 mL SDV) |
| Bulk Packaging / Sold As | As per distributor pack count |
| Shelf Life | Refer to vial label (typically 36 months) |
| Recommended Storage | Store at 20-25 °C (68-77 °F); protect from light; do not freeze |
| Latex Status | Not made with natural rubber latex |
| UNSPSC Code | 51422210 |
Key Features
-
Potent corticosteroid suspension (40 mg/mL) for anti-inflammatory use in acute settings
-
Formulated specifically for intramuscular or intra-articular injections
-
Single-dose vial helps minimize contamination risk
-
Contains benzyl alcohol preservative; proper use and contraindications apply
-
Brand-name quality from Bristol-Myers Squibb, recognized in specialty and hospital care