Product Specifications
| Specification | Details |
|---|---|
| Manufacturer | B. Braun Medical Inc. |
| Manufacturer # | E8000 |
| Brand | E³® |
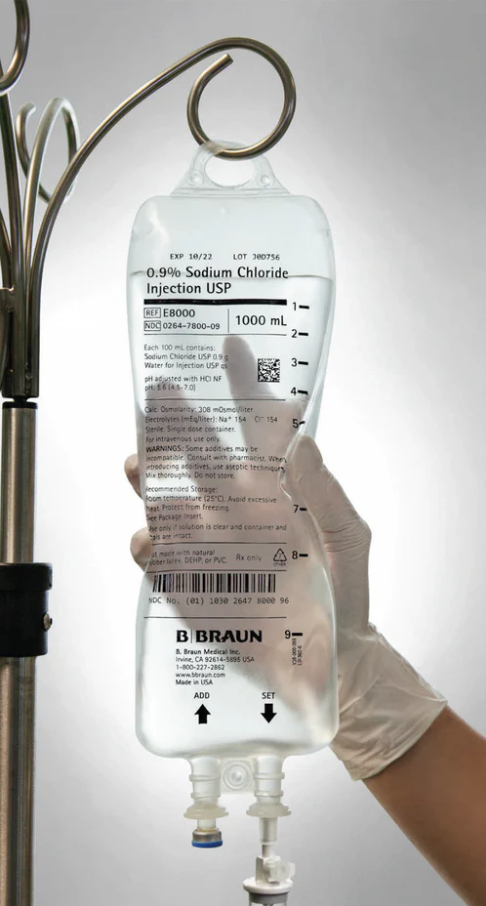
| Product Type | 0.9% Sodium Chloride Injection, USP (Normal Saline) |
| Container | Flexible IV bag (PVC- and DEHP-free) |
| Volume | 1,000 mL |
| Sterility | Sterile, single-use |
| Latex Status | Not made with natural rubber latex |
| Additive | None (Preservative-free) |
| Solution Type | Isotonic crystalloid solution |
| Port Type | Dual medication and IV ports |
| Packaging | Single bag / Case of 12 bags |
| Country of Origin | United States |
| Shelf Life | 30 months from date of manufacture |
| Recommended Storage | Store at 20° to 25°C (68° to 77°F); excursions permitted to 15–30°C (59–86°F) |
| UNSPSC Code | 51191602 |
Key Features
-
Phthalate- and PVC-Free Bag: E³ container eliminates PVC and DEHP exposure risks.
-
Sterile, Preservative-Free Solution: Ideal for fluid replacement and medication dilution — contains only USP-grade sodium chloride and water.
-
Physiologic Formulation: Isotonic 0.9% saline with specific gravity ~1.006 and pH 5.6; suitable for IV hydration and electrolyte maintenance.
-
Clinical Versatility: Indicated for fluid replenishment, treating metabolic alkalosis, and priming lines—a staple in inpatient and outpatient settings.
-
Dual Ports for Medication & IV Use: Enables efficient administration of fluids, drugs, and blood products.
-
Manufactured in the U.S.: Ensures compliance with domestic quality and manufacturing standards.
-
Packed for Enterprise Use: One-liter bags come in economically sized case packs of 12 for hospital and clinical fleets.