Product Specifications
| Specification | Details |
| Manufacturer / Brand | Long Grove Pharmaceuticals LLC |
| Manufacturer # / SKU | 81298-7620-3 (81298762003) |
| Product Type | Alkalinizing Agent / Injection Solution |
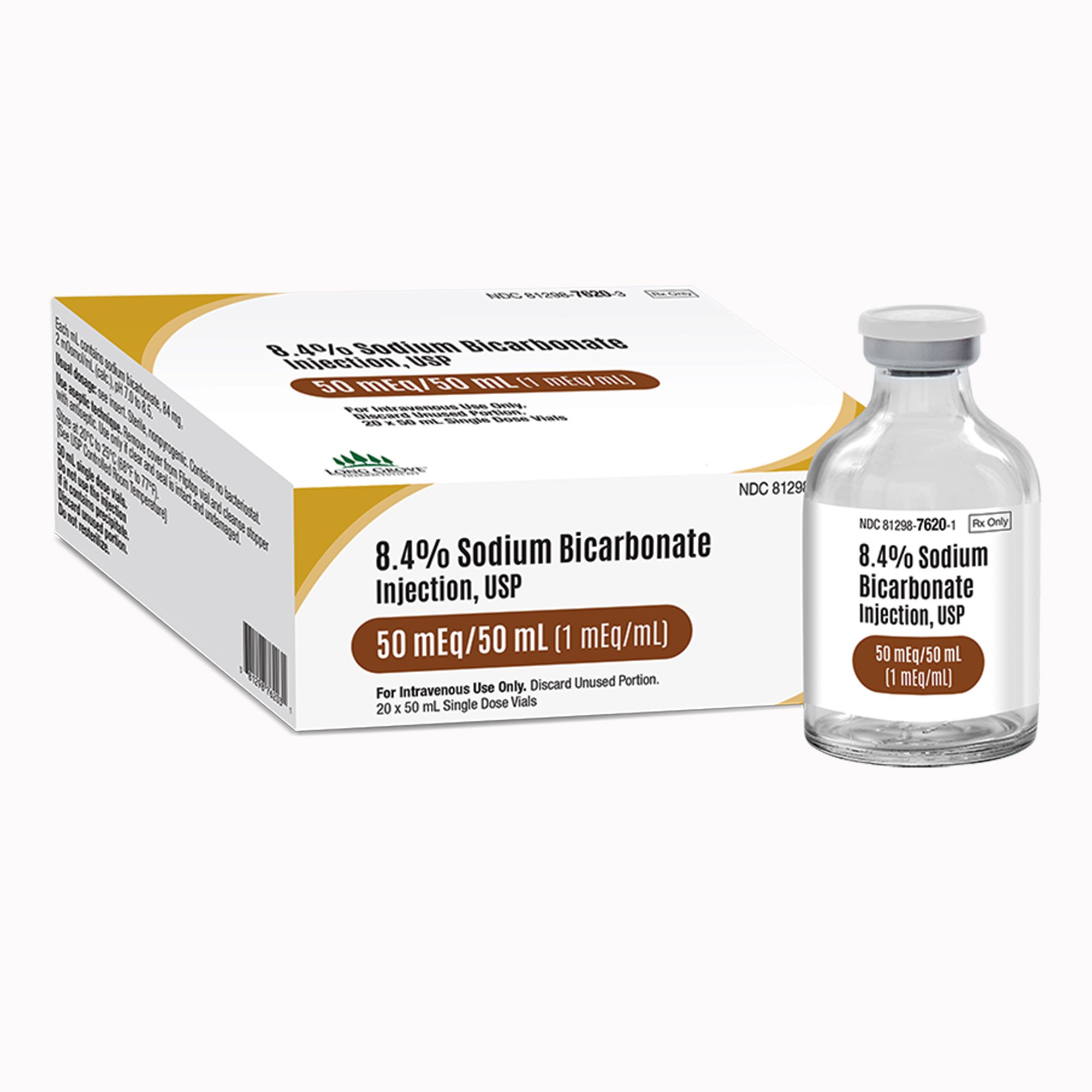
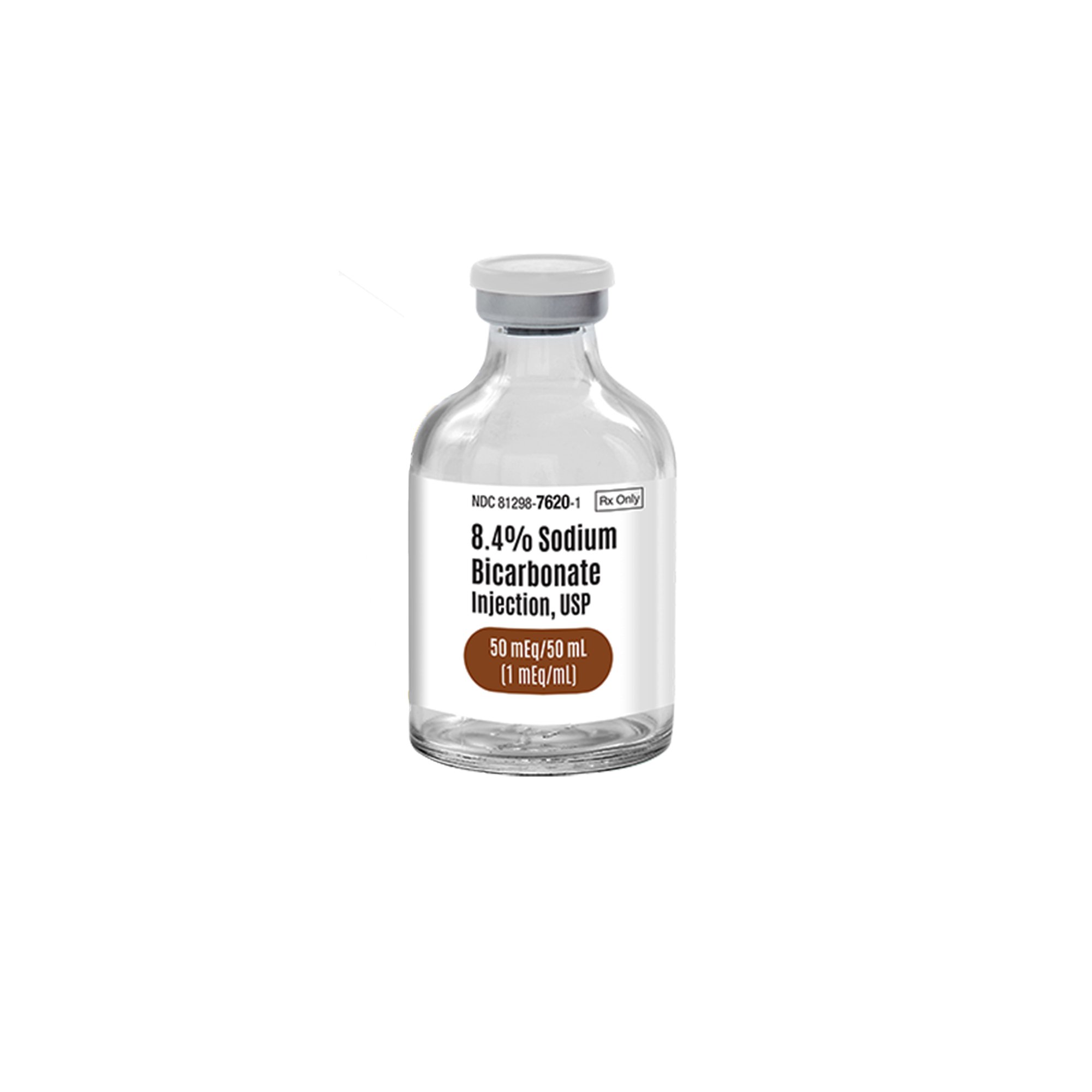
| Active Ingredient | Sodium Bicarbonate (NaHCO3) |
| Concentration | 8.4% (1 mEq/mL) |
| Total mEq | 50 mEq / 50 mL |
| Dosage Form | Injection, USP |
| Container Type | Single-Dose Vial (SDV) |
| Sterility | Sterile |
| Preservatives | Preservative Free |
| Latex Status | Vial Stopper Not Made with Natural Rubber Latex |
| Packaging / Sold As | 20 Vials per Carton |
| NDC Number | 81298-7620-3 |
| UNSPSC Code | 51191704 |
Key Features
-
Rapid Alkalinization: Essential for the immediate treatment of severe metabolic acidosis in critical care settings.
-
Optimal Concentration: Provides a potent 8.4% solution (1 mEq/mL) for effective restoration of plasma bicarbonate levels.
-
Single-Dose Safety: Packaged in a single-dose vial to maintain sterility and ensure the entire unused portion is discarded, minimizing contamination risk.
-
Preservative and Latex Free: Formulation is preservative-free and the vial stopper is not made with natural rubber latex, reducing potential patient sensitivities.
-
IV Use Only: Specifically indicated for intravenous administration, used frequently in cardiac arrest and other emergency medical protocols.